Talk to Our Scientists
"*" indicates required fields
Why do Free Sulfhydryl Analysis?
Disulfide bonds are crucial for the stability of the three-dimensional structure, the biological activity and the renaturation of the proteins. Cysteine is an amino acid that contains a sulfhydryl (-SH) group on its side chain, which can be oxidized to form these covalent disulfide bonds. Accurate characterization of both disulfide bonds and free sulfhydryl groups on a protein helps to understand the spatial structure of a protein, which provide the biological functions required. In providing full analytical characterization services for your protein samples, CovalX offers both direct Disulfide Bond Analysis and Free Sulfhydryl Analysis using mass spectrometry.
Characterizing Free Sulfhydryl Analysis using Mass Spectrometry
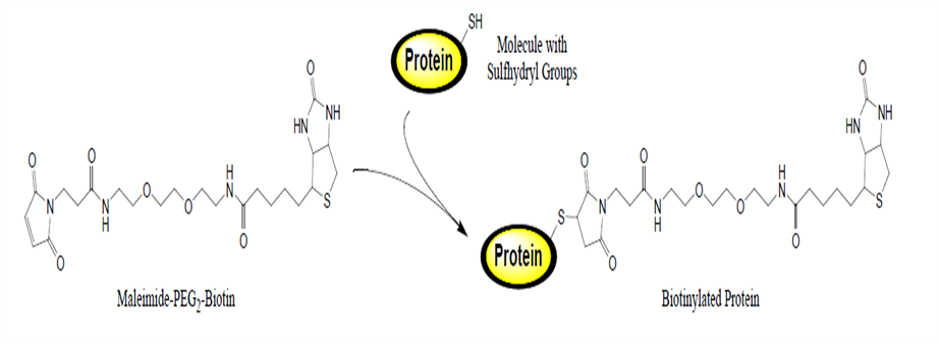
CovalX uses all the latest mass spectrometry hardware and software available on the market today. In order to accurately characterize free sulfhydryl, the samples are labeled with a maleimide derivative under acidic conditions in order to minimize the possibility of disulfide bond scrambling or non-specific binding.
In the above figure, NEM (N-ethylmaleimide) derivatization of a protein to obtain a biotinylated protein molecule at higher mass.
This maleimide derivatization can be conducted directly on the intact or on the reduced protein to localize the free sulfhydryls in different regions, such as on light versus heavy chain regions of a monoclonal antibody.
Free Sulfhydryl Analysis on intact Proteins
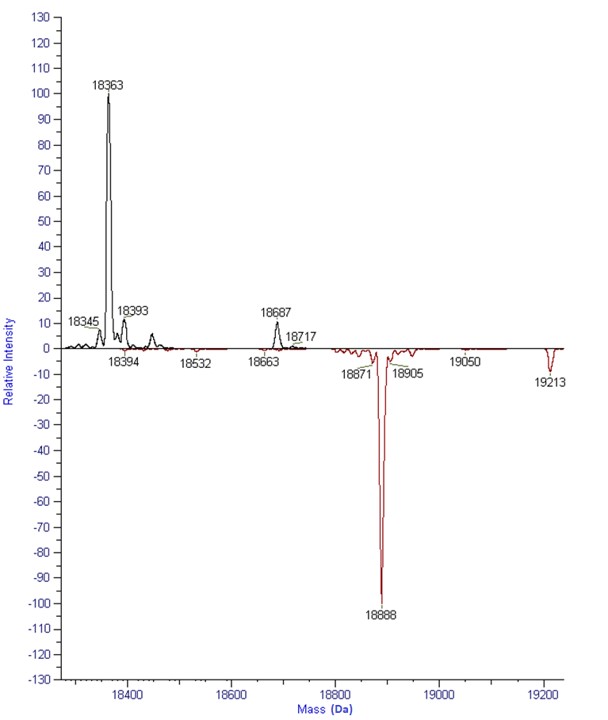
Free sulfhydryl analysis is important for the thorough characterization of all types of protein molecules. For some proteins, the analysis can be conducted on the intact protein without any need for protein reduction. It is shown below as an example of free sulfhydryl analysis on the intact Beta-Lactoglobulin A protein of 18.3 kDa. After protein labeling, the mass can be seen to shift exactly corresponding to 1 free sulfhydryl.
Monoclonal Antibody Free Sulfhydryl Localization
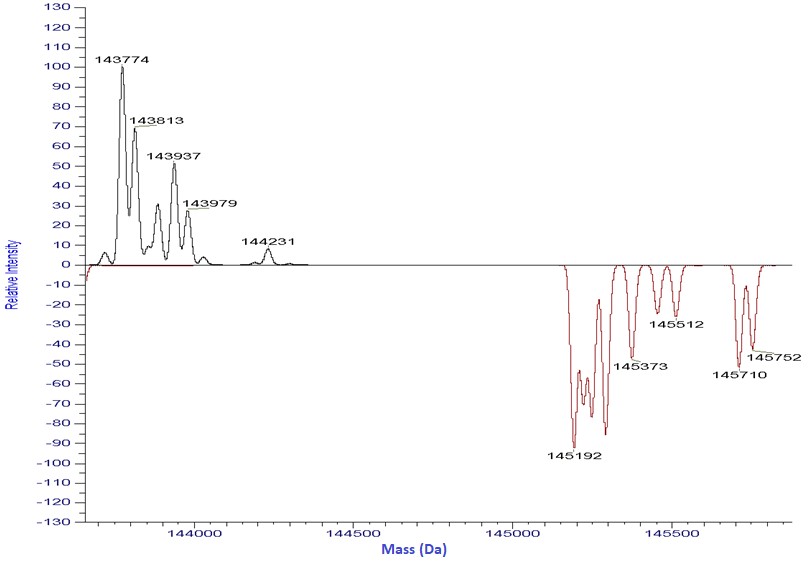
A typical IgG1 antibody will contain a total of 16 disulfide bonds including 4x interchain bonds in the hinge region and 12 bonds formed within individual domains of the protein. If these disulfide bonds are reduced as free sulfhydryls, this may have an effect on the stability of the molecule, increasing the aggregation or reducing the affinity. Free sulfhydryl analysis is an important step in properly characterizing a monoclonal antibody before release. It is shown below as an example of free sulfhydryl, that has been analyzed on both intact and reduced monoclonal antibodies.
For the intact mAb analysis, two total free sulfhydryls are detected. Further analysis after the reduction of the intact mAb into its light and heavy chain components, allows greater characterization and localization of the free sulfhydryls, as shown here.
By looking separately at the light and heavy chains of the molecule after reduction, it is possible to determine the free sulfhydryl location on the light chain specifically. Additional analysis at a peptide level can further localize the free sulfhydryl on the protein’s sequence.
Why Choose CovalX’s Free Sulfhydryl Analysis Services?
CovalX offers unique expertise in the field of free sulfhydryl analysis and advanced protein characterization, taking benefits of decades of experience in the field of mass spectrometry and large molecule analysis. With its team of experts and extensive experience in mass spectrometry, CovalX provides reliable results within a scheduled timeframe.
At CovalX, the free sulfhydryl analysis utilizes our extensive experience characterizing large molecules and biotherapeutics using mass spectrometry. CovalX uses all the latest instrumentation and software to provide the richest level of data possible for your protein. Having developed in-house both mass spectrometry instrumentation and characterization methods for the analysis of large molecules with mass spectrometry, CovalX can provide an unparalleled level of protein analysis.
What is needed from the customer?
Sample requirement
Typically 20-100 µg of recombinant proteins, purified proteins
Sequence information
Depending upon the level of data required, the sequence of the protein is not required to be provided. However, if it is not available from the client we can work to get this either from hybridomas sequencing or by De Novo Sequencing.
Turnaround time
Three weeks or less from receipt of sample material
Related services for protein interactions that CovalX provides
| Disulfide Bond Mapping |
| Intact Mass Analysis |
| Sequence Verification by PMF |
| Conformational/Protein dynamics (HDX) |
| Glycan Structure Determination |
| Aggregation Analysis |