Talk to Our Scientists
"*" indicates required fields
The analysis of the aggregation phenomenon from therapeutic proteins is of crucial importance as more and more pharmaceutical products are proteins or peptides. Aggregation can not only result in reduced efficacy of biopharmaceuticals but also increases the risk of inducing an immune response to the mAb rather than the pathogenic antigen it was supposed to detect. Aggregation is even specifically listed as one of the key factors to monitor within the ICH Q6B guidelines of biologics. CovalX offers a unique technique for the direct characterization of therapeutic protein samples based on High-Mass MALDI mass spectrometry. This technique is often utilized orthogonally to SEC, SEC-MALS or AUC when a discrete measurement of the aggregates is desired. The MALDI mass measurement provides direct detection of the individual species present, not an average as seen by many techniques.
 Graph Aggregates1
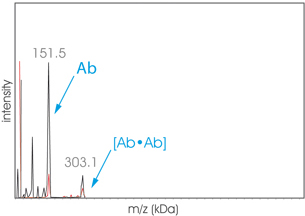
Graph Aggregates1 Therapeutic Antibody Aggregates
High-Mass MALDI TOF analysis (CovalX HM) of an aggregated sample of the therapeutic protein Hab41 before (black) and after (red) cross-linking. The sample has been submitted to the SEC before cross-linking protocol with the CovalX K200 MALDI MS Stabilization Kit. (Contact Us to learn more about this Kit)
The fraction analyzed exhibit a dimer (303 kDa). The high-mass analysis allows distinguishing between covalent and noncovalent aggregation.
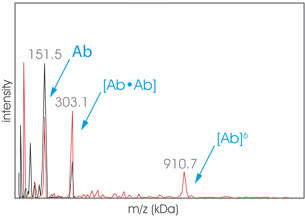 Graph Aggregates2
Graph Aggregates2 High Mass MALDI ToF analysis of an aggregated sample of therapeutic protein Hab42 before (black) and after (red) cross-linking. The sample has been submitted to SEC before cross-linking protocol using the CovalX K200 MALDI MS Stabilization Kit. The fraction analyzed exhibits a dimer (303 kDa) and hexamer (910 kDa).
For more details concerning protein aggregates analysis, contact us using the form on this page.
 CovalX Interaction Analysis
CovalX Interaction Analysis CovalX Biotherapeutic Lab Services
CovalX Biotherapeutic Lab Services