Talk to Our Scientists
"*" indicates required fields
Why Choose CovalX’s SPR Services?
CovalX has decades of experience analyzing protein interactions and protein complexes in the field of therapeutic protein development. CovalX has several unique technologies and applications using mass spectrometry which can provide a unique characterization of monoclonal antibodies. Surface Plasmon Resonance (SPR) provides an added benefit to our characterization capabilities offering high-throughput, real-time, label-free affinity and kinetic characterizations on a range of sample types including monoclonal antibodies, proteins, and small molecules.
At CovalX we utilized the latest SPR instrumentation and software with experts trained in the sample preparation, analysis and data processing required. Although related to biolayer interferometry (BLI), the SPR methods can provide higher accuracy and utilize lower amounts of materials than BLI. With its team of experts and extensive experience in protein interaction analysis, CovalX provides reliable results within a scheduled timeframe.
Analysis Services offered with SPR
Services: Drug discovery, Lead optimization, Target characterization
Drug Discovery:
CovalX provides the following antibody-related services via SPR analysis:
- Monoclonal antibody pairing by “epitope binning” to identify and group different mAbs(to enable antibody pairing according to epitope binding on the same antigen)
- Full kinetic analysis/ ranking by on-rates (ka), off-rates (kd), and affinity constants (KD)
- Antibody screening against label-free antigen directly in solution
Common Ligand to Analyte Assay Formats:
- Antigen and Antibody Fragment Characterization
- Protein to Protein or Peptide Interactions
- Protein to DNA or RNA Interactions
- Protein to Lipid Interactions
- Protein to Small Molecule Interactions
Type of analyzed data available:
- Kinetics/affinity characterization or screening (Ka/Kd)
- LMW interaction analysis
- Fragment screening,
- Immunogenicity
- Concentration analysis
- Thermodynamics,
- Comparability
Additionally, CovalX offers unique Mass Spectrometric based characterization of protein interactions through our Intact Protein Complex Analysis (Stoichiometry, exact mass analysis) and high-resolution Epitope Mapping (XL-MS or HDX-MS Analysis)
Lead Optimization:
A lead compound’s pharmacokinetics and pharmacodynamics (PK/PD) are directly affected by the compound-target on-rate (Ka) and off-rate (Kd). Kinetic evaluation of a lead molecule by SPR ensures in vivo selection of compounds on conditions that are related to target binding and target selectivity. Characterization of biotherapeutic candidates and label-free screening using SPR are able to adjust early in vitro ADME analyses.
Additionally, CovalX offers a more in-depth characterization of lead compound binding sites through our HDX-MS analytical service.
Target and Compound Characterization:
Drugs targeting membrane-bound proteins continue to be a large focus for research and development. SPR can offer a unique solution to measure these challenging proteins in their native lipid states. Rapid characterization of the interactions for these drug compounds with a target protein is very crucial before optimization when hundreds of potential ‘lead compounds’ must be researched. SPR offers an approach that enables the direct determination of the interactions between unlabeled compounds and the targets, providing a trusted and rapid method for compound characterization.
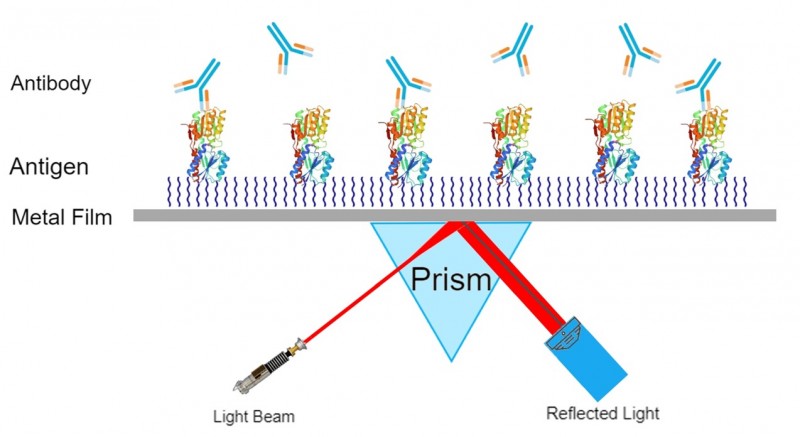
How SPR Works
Unlike mass spectrometry which measures the mass of molecules, SPR measures molecular adsorption on a surface. This adsorption can be due to binding or interaction of protein interactions such as ligand binding, or in the case of antibodies, binding their target antigen.
SPR characterization has become an established analysis technique within the biotechnology and pharmaceutical industries. This technology can be applied to identify the interactions of almost any molecular system. SPR can detect minute changes in the refractive index of plasmon waves close to the surface through which they pass. SPR provides a real-time analysis with no sample labeling required. The absence of sample labeling decreases the time demanded to prepare samples for determination and eliminates the concern that a tag may influence the reaction. Real-time monitoring makes it feasible to measure detailed information about binding events, including the association and dissociation reaction kinetics.
Why is CovalX’s SPR Services the Best Option?
| Expertise | Experienced team analyzing protein complexes in the industry for more than 15 years. |
|---|---|
| Latest Equipment | CovalX uses only the latest SPR instrumentation and software. |
| Quality Work | Advanced initial intact screening of proteins helps provide the most reliable rate of success. |
| Affordable | Competitive cost compared with other technologies. |
| Low Sample Consumption | Only 75μg of each protein is required. |
Timeline, Sample Requirements and Resolution
- Sample Consumption: 75ug of each binding protein.
- Cost: CovalX offers upfront competitive pricing on a per-project basis.
- Affinity range: fM to mM binding
- Dissociation rate constant (kd): 10-5 to 1 s-1
- Sample type: Small-molecule drug candidates to high-molecular-weight proteins (also DNA, RNA, polysaccharides, lipids, cells, and viruses) in various sample environments (e.g., in DMSO-containing buffers, plasma, and serum)
Notable Benefits with CovalX SPR Services
- Initial Mass Spectrometric Characterization of intact protein provides robust screening before beginning interaction experiments. This is unique to CovalX services.
- Expertise: CovalX has over a decade of experience working and analyzing protein interactions using mass spectrometry.
- Latest Equipment: CovalX utilizes the latest SPR instrumentation and analysis software available.
- The interaction is analyzed in solution in native conditions, with the accuracy of SPR.



 CovalX Surface Plasmon Resonance (SPR) Services Brochure
CovalX Surface Plasmon Resonance (SPR) Services Brochure