Talk to Our Scientists
"*" indicates required fields
ICH Q6B, the International Council on Harmonization Quality recommends test procedures and acceptance criteria for biological products. These references outline the physicochemical characterization, binding and biological activity, quantity, and purity of a biological product. These guidelines have been accepted as standard practice by the FDA, EMeA, and Japanese regulatory agencies, including many others. They describe a physicochemical characterization program which will generally include composition determination, physical properties, and structure of the therapeutic protein.
Why Choose CovalX’s Characterization Services?
CovalX has long been established as a leader for the characterization of therapeutic proteins such as monoclonal antibodies. CovalX offers a complete solution to provide characterization following the ICH Q6B guidelines established for IND submission. CovalX’s is now offering:
Structural Characterization and Confirmation:
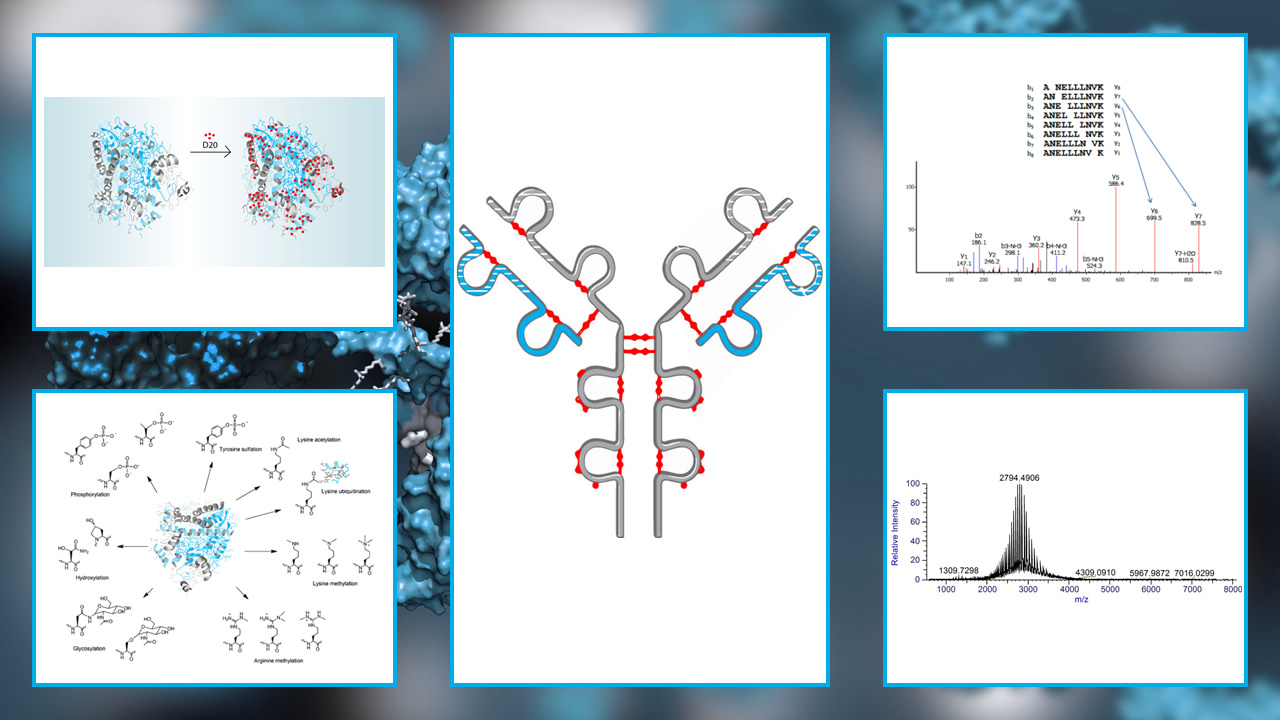
Complete Amino Acid Sequencing and Amino Acid Composition
Peptide map
Sulfhydryl group(s) and disulfide bridges
Carbohydrate structure
- Monosaccharide composition (Neutral and Amino monosaccharides, Sialic Acid)
- Oligosaccharide linkage patterns (antennary profile)
- Glycosylation sites of polypeptide chain
Physicochemical Properties:
Molecular Weight or size
- Intact Mass (LC-MS)
- Size exclusion chromatography (SEC)
Isoform and Electrophoretic Pattern
- SDS-PAGE
Extinction Coefficient (or molar absorptivity)
- UV/visible spectrophotometry (280 nm)
Liquid chromatographic patterns
- Size Exclusion Chromatography (SEC)
- Reverse-phase HPLC
- Cation-Exchange Chromatography (CEX)
Spectroscopic profiles
- Circular dichroism (CD)
- Ultraviolet and visible absorption (UV-Vis)
Impurities:
Truncated forms
- HPLC
- SDS-PAGE
- Peptide mapping
Other modified forms:
- Deamidated
- Disulfide scrambling
- Oxidization
- Glycosylation
Aggregates:
- Size Exclusion MultiAngle Light Scattering (SEC-MALS)
- Analytical UltraCentrifugation (AUC) for Aggregation
- Capillary Electrophoresis (CE)
Fourier transform infrared (FTIR) spectroscopy
Thermal Stability
- Differential Scanning Calorimetry (DSC) Analysis